Carbon Monoxide Dot Structure
A step-by-step explanation of how to draw the CH4N2O Lewis Dot Structure (Urea).For the CH4N2O structure use the periodic table to find the total number of v.

Lewis Dot Diagram Worksheet
A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon monoxide ).For the CO structure use the periodic table to find the total number.
14+ Co Lewis Dot Structure Robhosking Diagram
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures
Lewis Dot Diagram For F Drivenheisenberg
Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure.

Lewis Structure for CO (Carbon Monoxide) Carbon monoxide, Ap
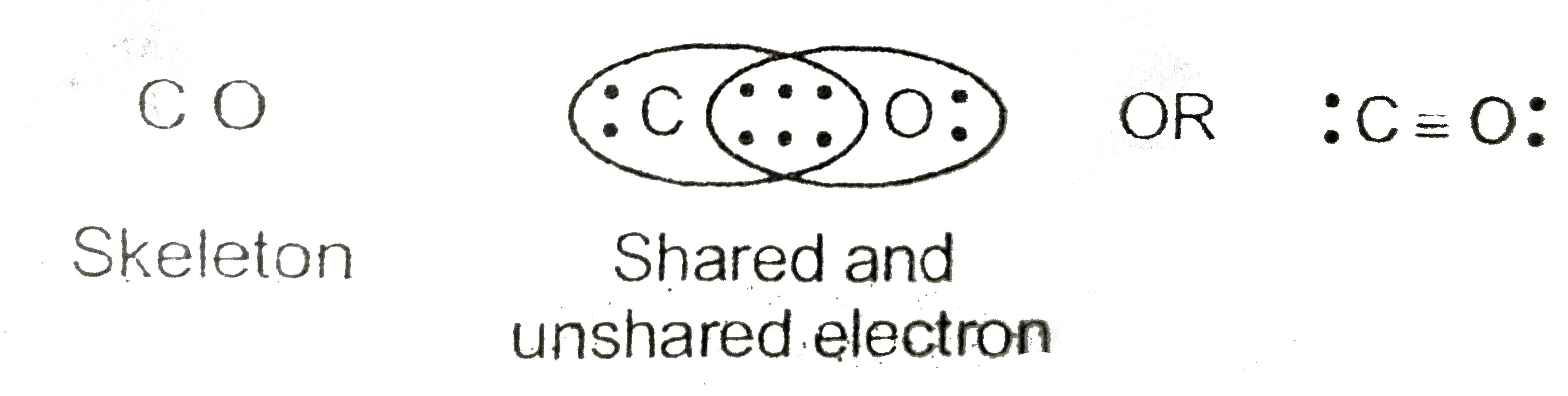
For the CO Lewis structure there are a total of 10 valence electrons available. Transcript: This is the CO Lewis structure: Carbon monoxide. We have 4 valence electrons for Carbon and 6 for Oxygen, for a total of 10 valence electrons. So we have a Carbon and an Oxygen atom bonded together. We'll put 2 electrons between the atoms to form a.
Lewis dot structures YouTube
When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight valence electrons (an octet). Created by Sal Khan. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Peter Patterson 3 years ago
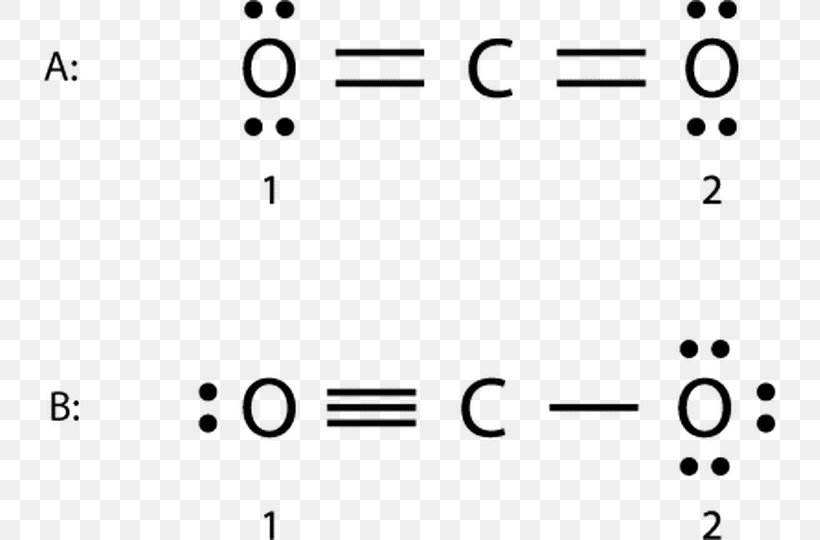
Formal Charge Lewis Structure Resonance Chemistry Carbon Dioxide, PNG
The Lewis Structure (Lewis Dot Diagram) for CO. 1. Count electrons 2. Put least electronegative atom in centre.more.more The Lewis Structure (Lewis Dot Diagram) for CO.1..

CO (Carbon Monoxide) Lewis Dot Structure with Formal Charge
Step #1: Calculate the total number of valence electrons. Here, the given molecule is CO (carbon monoxide). In order to draw the lewis structure of CO, first of all you have to find the total number of valence electrons present in the CO molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
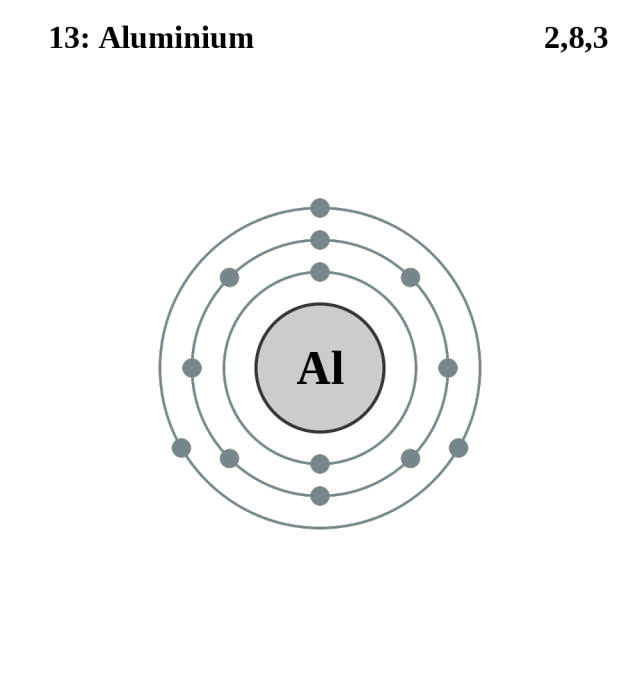
Lewis Dot Diagram For Al Wiring Diagram Source
How to Draw the Lewis Dot Diagram for Carbon monoxide (CO) It is helpful if you: Try to draw the CO Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to CO for more practice. List of Lewis Structures Lewis Structures for CO.
:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
Electron Dot Structure For Fluorine
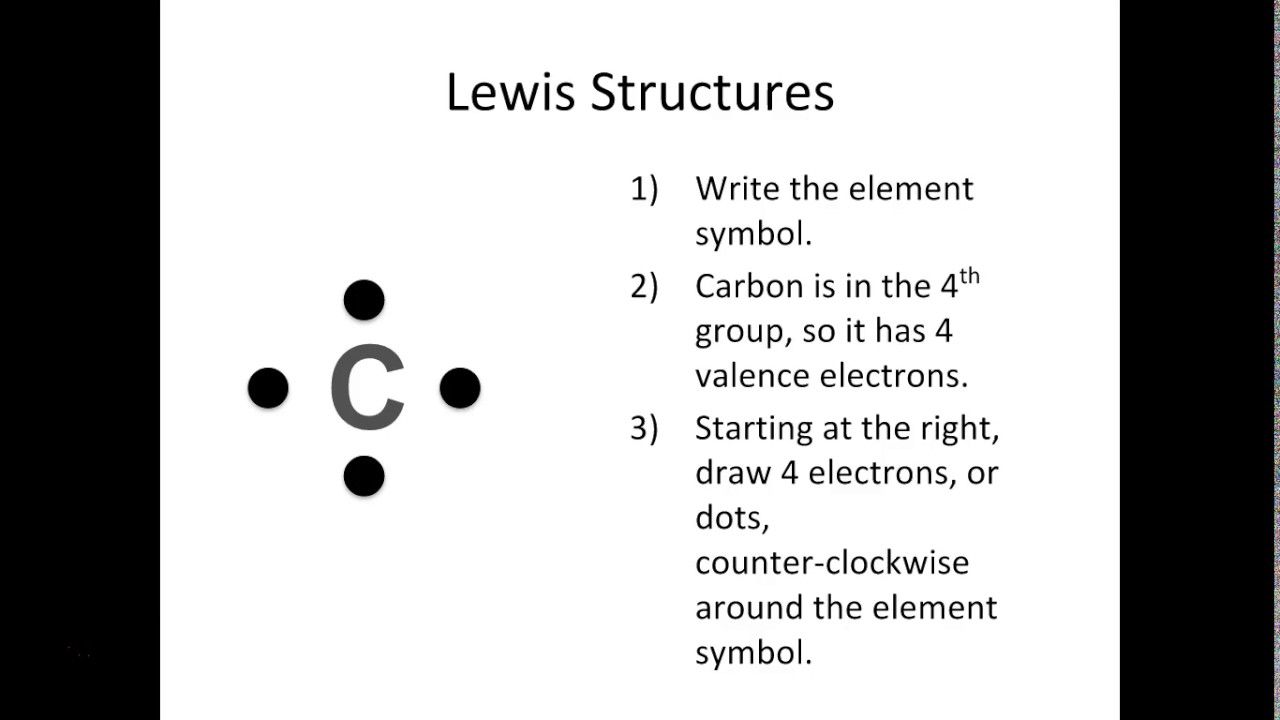
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
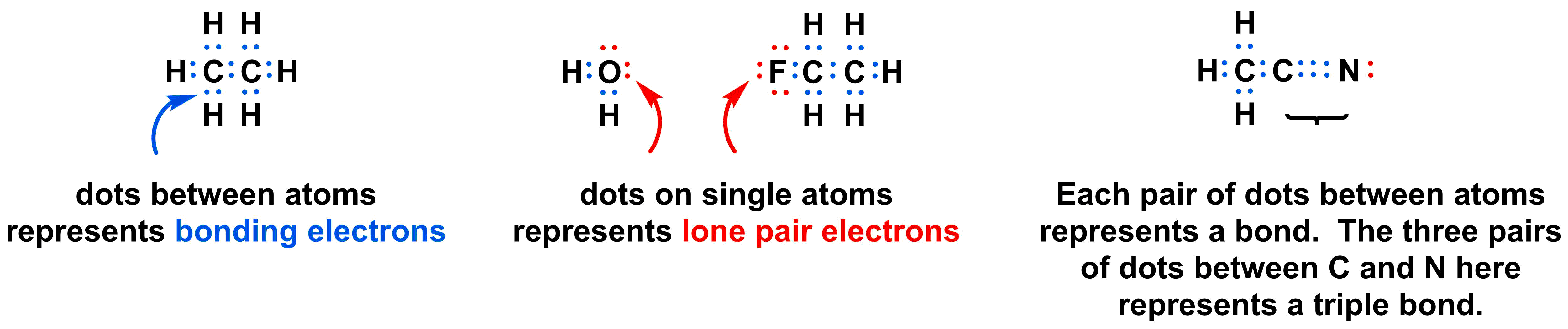
Lewis dot structures of atoms and ions pogil answers
That is CO. Carbon has 4 valence electrons and Oxygen has 6. In total, there are 10 valence electrons. I realize that explaining how to draw the lewis dot structure in words might get confusing so what I'm going to do is put a picture of what the diagram will look like along with a video showing step by step on how to exactly create the diagram.
CO Lewis Structure How to Draw the Dot Structure for CO YouTube
A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon dioxide). For the CO structure use the periodic table to find the total number of valence electrons for t.more.
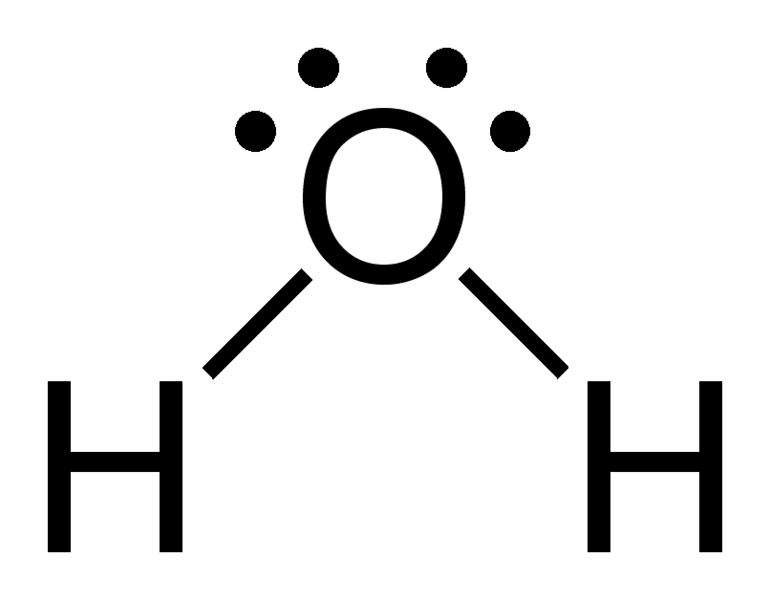
Laura September 2010
The Lewis Dot Structure (Lewis Dot Diagram) of or for CO.1.Count valence electrons of CO2.Keep the least electronegative atom in centre3.Put one valence e.
Lewis Dot Structure Generator demorejaz
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4.2 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.
9.1 Chemical Bonds, Lewis Symbols, and the Octet Rule Chemistry
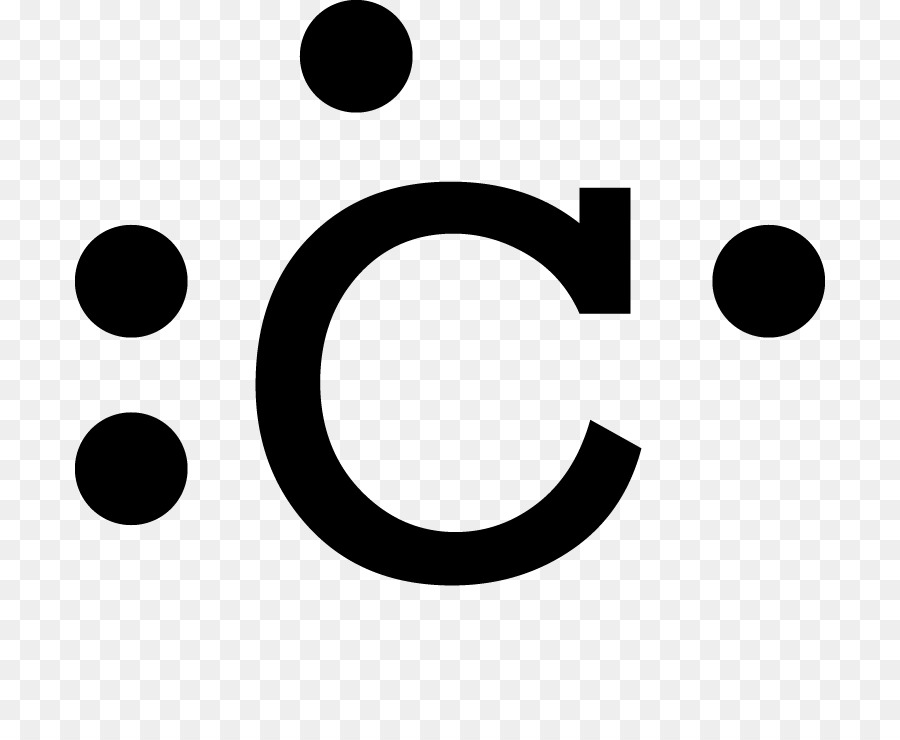
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.

CO (Carbon Monoxide) Lewis Dot Structure with Formal Charge
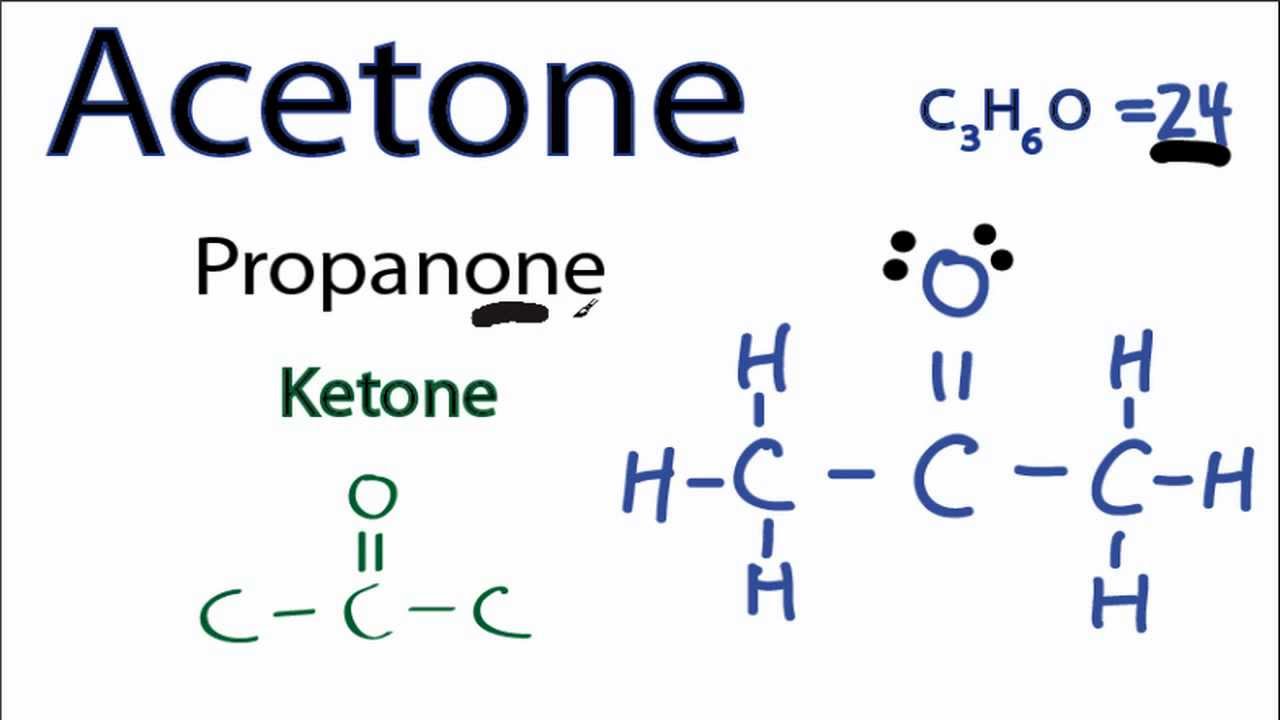
The Lewis structure, also called as electron dot structure, is a simplified method of representing the number of valence electrons present within an atom or a molecule. Furthermore, the structure helps with determining the number of lone pairs of electrons present in an atom and how they act in a bond formation.